
Nonetheless, we will attempt a heuristic argument to make the result at least plausible. Any rule that might be capable of predicting the allowed energies of a quantum system must also account for the wave-particle duality and implicitly include a wave-like description for particles. Moreover, it relies heavily on classical ideas, clumsily grafting quantization onto an essentially classical picture, and therefore, provides no real insights into the true quantum nature of the atom.
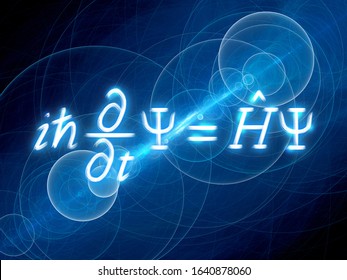
While the Bohr model is able to predict the allowed energies of any single-electron atom or cation, it by no means, a general approach. The Schrödinger Equation: A Better Approach There is no rigorous derivation of Schrödinger’s equation from previously established theory, but it can be made very plausible by thinking about the connection between light waves and photons, and construction an analogous structure for de Broglie’s waves and electrons (and, later, other particles). This was a direct challenge to Schrödinger, who spent some weeks in the Swiss mountains working on the problem and constructing his equation.

Schrödinger gave a polished presentation, but at the end Debye remarked that he considered the whole theory rather childish: why should a wave confine itself to a circle in space? It wasn’t as if the circle was a waving circular string, real waves in space diffracted and diffused, in fact they obeyed three-dimensional wave equations, and that was what was needed.

Shortly after it was published in the fall of 1925 Pieter Debye, Professor of Theoretical Physics at Zurich and Einstein's successor, suggested to Erwin Schrödinger that he give a seminar on de Broglie’s work. To be introduced to the general properties of the Schrödinger equation and its solutions.ĭe Broglie’s doctoral thesis, defended at the end of 1924, created a lot of excitement in European physics circles.


 0 kommentar(er)
0 kommentar(er)
